High-throughput Phenotyping towards Herbicide discovery
The Role of Chlorophyll Fluorescence in Herbicide Screening
The global challenge of herbicide resistance, coupled with environmental concerns, has intensified the demand for innovative, sustainable, and effective herbicides. Traditional herbicide discovery methods are often slow, resource-intensive, and environmentally taxing. Multichannel plant imaging, for example chlorophyll fluorescence imaging, can offer a robust indicator of plant health and stress responses. Plant imaging offers rapid, quantitative, and high-throughput screening of novel herbicidal compounds. This approach not only accelerates the identification of promising candidates but also minimizes environmental impact by reducing the need for extensive field trials.
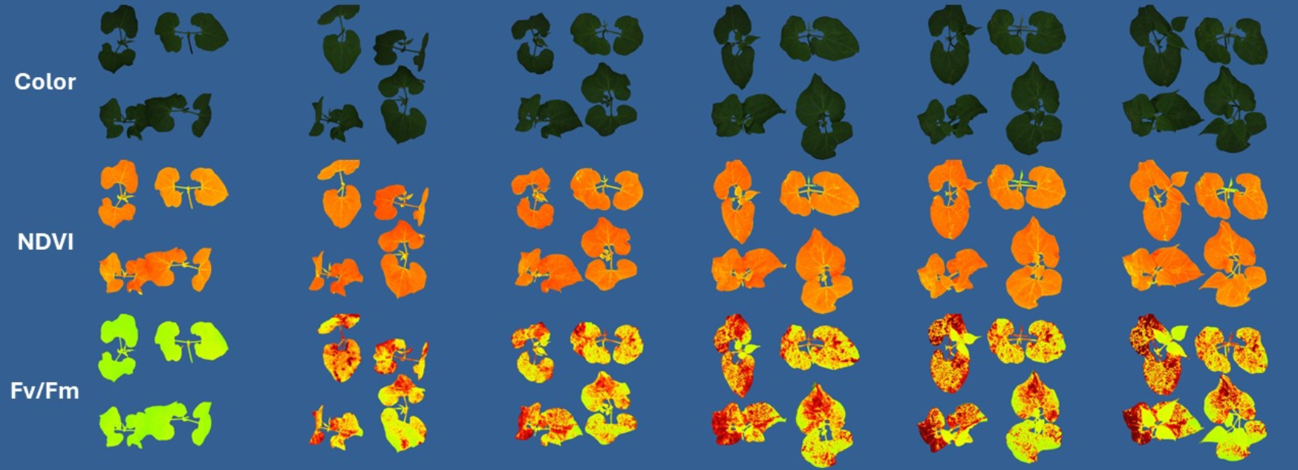
Chlorophyll fluorescence, particularly the Fv/Fm parameter, quantifies the maximum quantum efficiency of photosystem II (PSII). Herbicides that disrupt photosynthesis induce a measurable decline in Fv/Fm, making it a powerful early marker of herbicidal activity. This method is highly sensitive, non-destructive, and capable of detecting stress responses before visible symptoms appear.
A Case Study
A recent study conducted at Ghent University (Backx et al., 2024; Backx et al., 2025), using the CropReporter system, demonstrated the efficacy of plant imaging in screening novel herbicidal compounds. The researchers focused on 3-acyltetramic acids and their prodrugs, which are biologically derived and show promise as sustainable herbicides. Leaf disks were obtained from fully developed leaves of tomato plants (Solanum lycopersicum ‘Moneymaker’ L.), cultivated under controlled greenhouse conditions (21 °C, LED lighting, and regular fertilization). Standardized disks (0.6 cm or 1.1 cm diameter) were punched from leaves and placed in 24- or 96-well plates containing sterile distilled water for a destressing period prior to treatment. Fv/Fm measurements were taken at 24, 48, 72, 96, and 144 hours post-treatment. IC₅₀ values (the concentration required to reduce Fv/Fm by 50%) were calculated using four-parameter log-logistic models.
Key Findings
The study demonstrated that Fv/Fm imaging provides a fast, reliable, and quantitative method for herbicide screening. Compounds inducing strong reductions in Fv/Fm corresponded with high herbicidal activity, validating the use of this parameter as a proxy for phytotoxicity. The approach also enabled early detection of herbicidal effects with minimal compound use, quantitative potency ranking, and structure-activity analysis.
While in vitro assays provide valuable insights, translating these results to field conditions requires careful consideration. Some prodrugs, which showed promising activity in leaf disk assays, did not outperform the original compound in spray tests on Amaranthus retroflexus seedlings. Targeted field validations are still needed to ensure real-world efficacy. However, high-throughput phenotyping does reduce the time and cost associated with herbicide development, enabling researchers to focus on the most promising candidates. And, by minimizing the use of compounds and reducing reliance on field trials, the approach aligns with the principles of sustainable agriculture. The integration of phenotyping data with other omics technologies (e.g., genomics, metabolomics) can further refine the understanding of herbicide modes of action and resistance mechanisms.
Conclusion
By using multispectral plant imaging parameters in herbicide discovery as a quantitative proxy for plant health, researchers can rapidly screen novel compounds, optimize their efficacy, and reduce environmental impact. As demonstrated by the work at Ghent University, this approach not only benefits early-phase herbicide discovery. It may also pave the way for more sustainable and effective weed management strategies.
For further details, please explore the original research publications:
- Backx, S., et al. (2024). Synthesis of Mixed Phosphonate Esters and Amino Acid-Based Phosphonamidates, and Their Screening as Herbicides. International Journal of Molecular Sciences. DOI: 10.3390/ijms25094739
- Backx, S., et al. (2025). Synthesis and Herbicidal Assessment of 3-Acyltetramic Acid Prodrugs. ACS Omega.
DOI: 10.1021/acsomega.5c02851

