Introduction to Chlorophyll Fluorescence
In agriculture and horticulture, optimizing photosynthesis and preventing stress (e.g. ensuring adequate water supply, necessary nutrients, and favorable conditions) are essential for maximizing crop yield. Moreover, efficient light capture and energy conversion are key to healthy plant growth and high productivity. Technological advancements now enable growers and researchers to quantify and monitor plant processes, for instance photosynthetic activity, stomatal conductance, and transpiration, with greater precision, providing insights into plant functioning that were previously difficult to achieve. One particularly influential technology in plant sciences is the analysis of chlorophyll fluorescence.
But what exactly is this fluorescence signal, and why is it used so often in plant science?
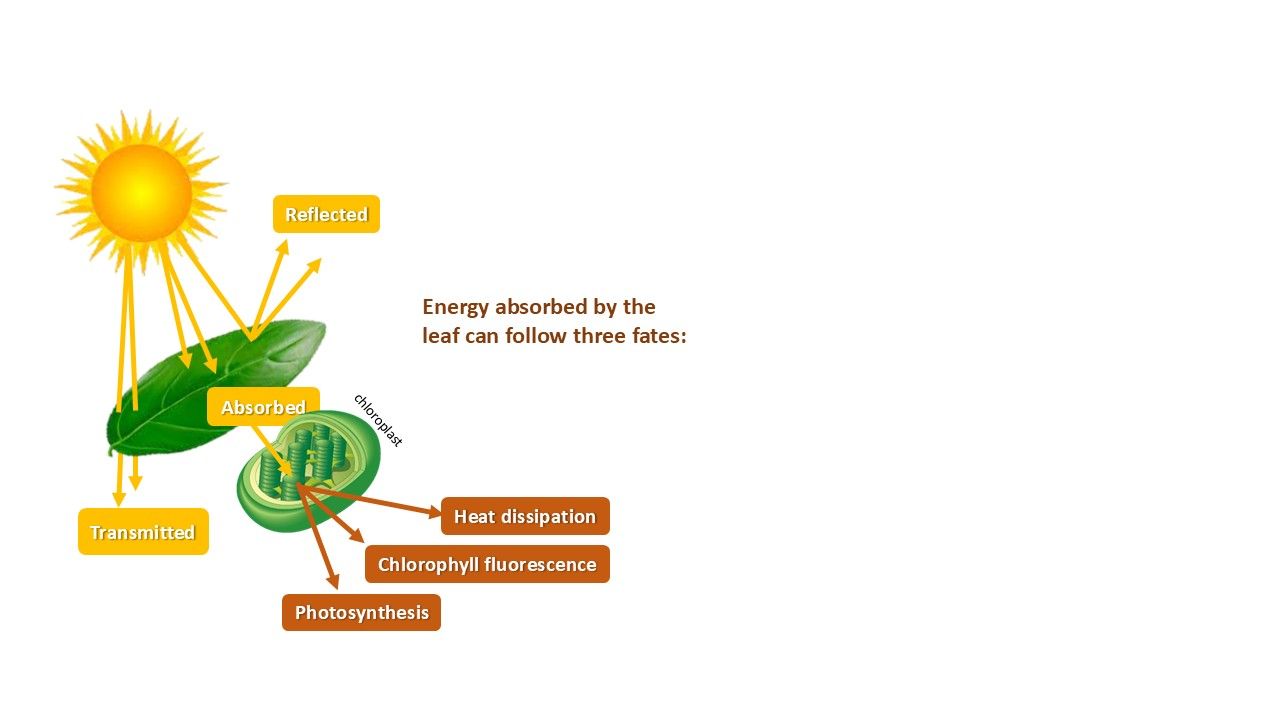
When a plant is illuminated, chlorophyll molecules—particularly the photosystems within—absorb light. While most of this energy is used for photosynthesis, some is dissipated as heat, or re-emitted as fluorescence (Figure 1). Unlike reflected light, fluorescence is emitted by the plant itself, effectively making the plant 'giving off' light (Figure 2).

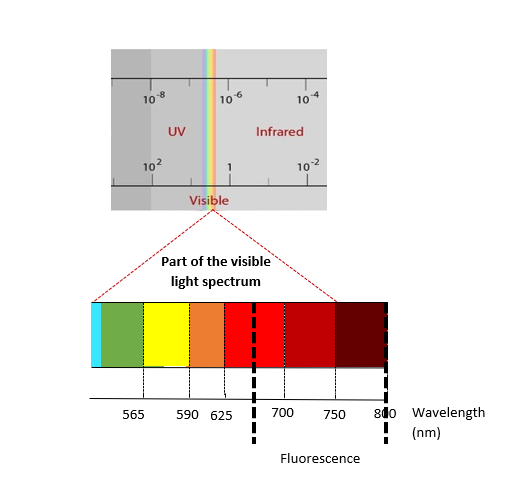
Chlorophyll fluorescence is emitted at relatively long wavelengths at the edge of the visible light spectrum, with peaks of emission at 680 and 730 nm (Figure 3). It originates from the plant's photosystems, which are located in the thylakoid membrane of the chlorophyll molecules. The photosystems are involved in capturing light, and driving photosynthesis.
Basis of Chlorophyll Fluorescence Measurements
To understand how chlorophyll fluorescence analysis works, we need to explore the basis of photosynthesis.
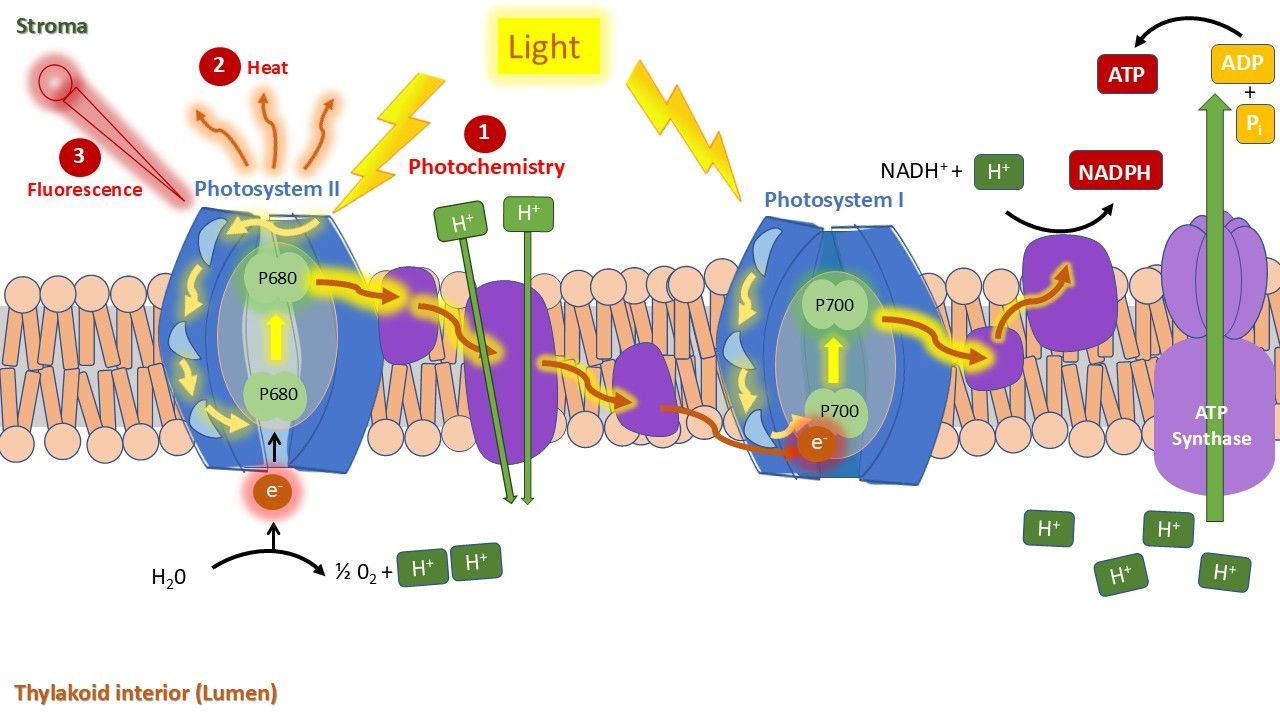
As seen, fluorescence is emitted from the chlorophyll molecules, specifically the protein complexes known as photosystems I and II, which are embedded in the thylakoid membranes (Figure 4).
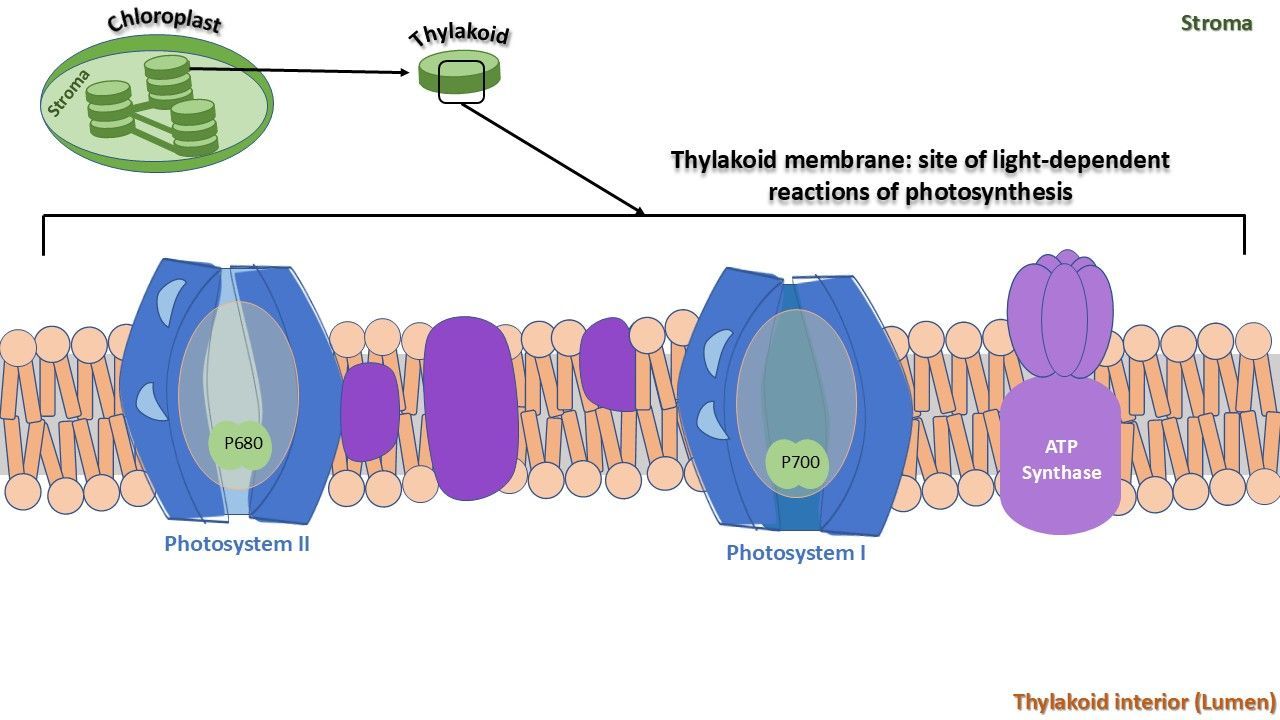
Photosynthesis processes are split in two phases:
• The light phase
• The dark phase (also called Calivn cycle)
When we are looking at chlorophyll fluorescence, we are looking at the
light dependent phase of photosynthesis.
During the light-dependent phase, energy from light is converted into chemical energy, as a way to store it. When the photosystems capture light through their antennae, their reaction centers become excited. This energy must be effectively managed by the plant, otherwise it can react with molecular oxygen, generating an excited state of oxygen known as 'reactive oxygen species' or ROS, which are harmful and can cause cellular damage. Hence, plant processes to safely direct the energy operate at high speeds. The transfer of energy from excited-state chlorophyll to photochemistry or heat dissipation is referred to as quenching.
The light energy captured by the photosystems is used to carry electrons through the electron transport chain across the thylakoid membrane (Figure 5). The process establishes a hydrogen proton gradient, which powers the enzyme ATP synthase to produce ATP. Simultaneously, light energy enables the formation of NADPH. The energy stored in ATP and NADPH is subsequently utilized in the Calvin cycle (dark phase) to fix CO₂ and synthesize glucose.
This entire process occurs at a specific rate (though a fast one), which limits the proportion of absorbed light energy that can be allocated exclusively to photosynthesis. As previously mentioned, some of this energy is dissipated through other pathways: as heat or fluorescence.
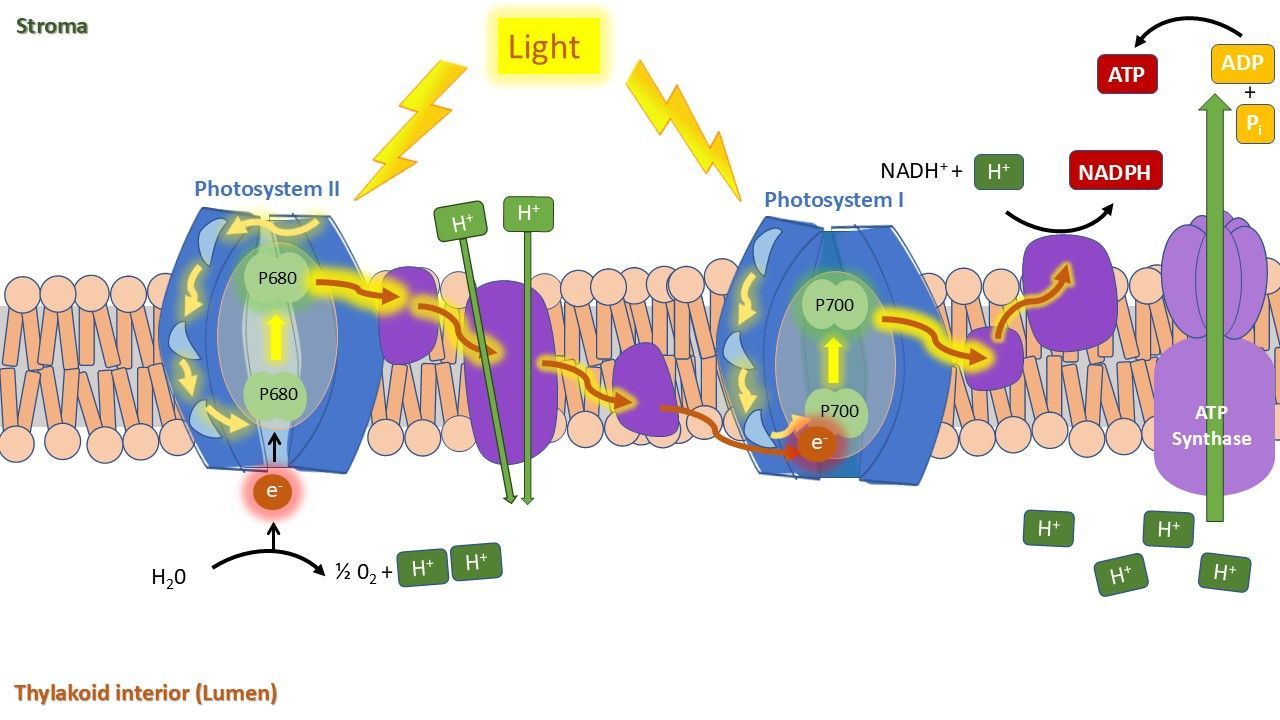
Energy dissipation through heat and fluorescence happens continuously (Figure 6). To prevent photo-damage, the plant tries to actively regulate this energy release via a process known as non-photochemical quenching (NPQ). NPQ represents the dissipation as heat, in a controlled way. Additionally, the excited chlorophyll molecules release some of the absorbed energy as photons, which is the fluorescence. Fluorescence is primarily emitted by photosystem II and accounts for only a small fraction of the absorbed energy (approximately 1-2%).
The different energy pathways are in direct competition with each other, meaning that an increase in one pathway leads to a decrease in the others. This competition enables us to calculate and quantify how a plant balances energy absorption, conversion, and dissipation, providing valuable insights into the plant's physiological functioning.
Why does fluorescence yield change?
When a plant is transferred from dark to light, fluorescence emission increases sharply within the first few seconds (Figure 7). Understanding what happens inside the chlorophyll molecules during this transition is crucial.
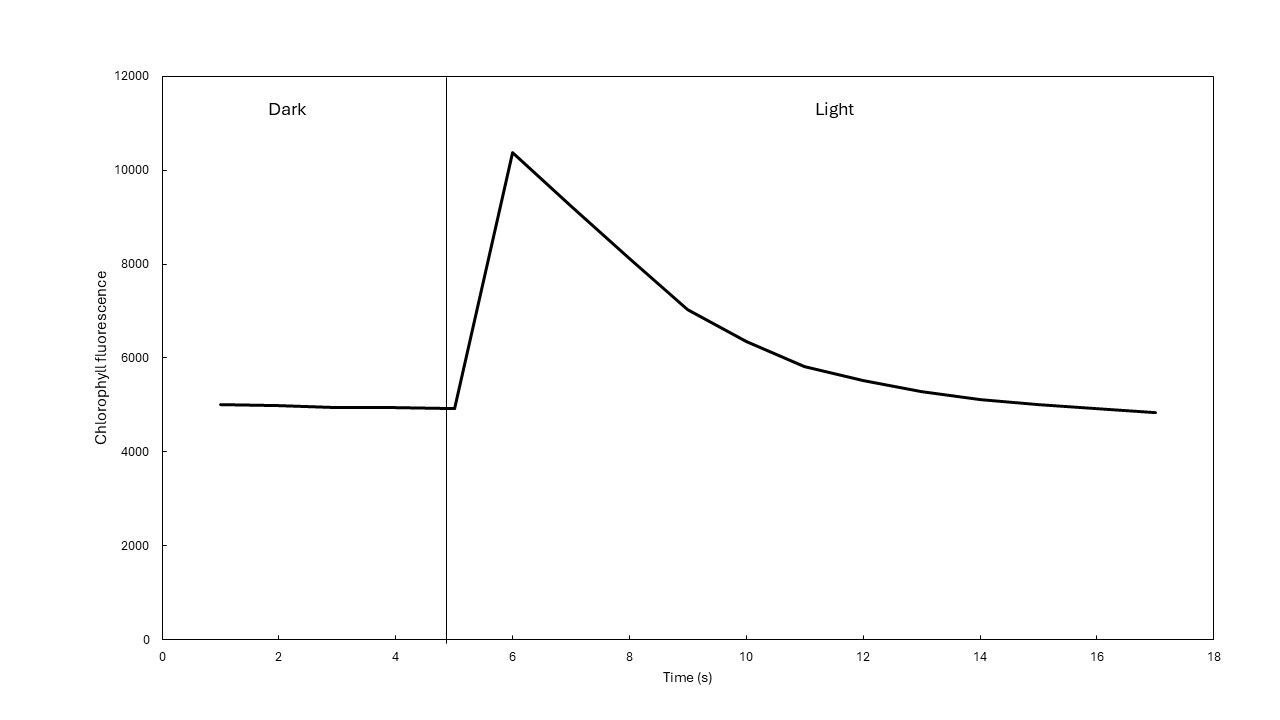
The initial rise in fluorescence yield during the first few seconds after light exposure has been explained by a reduction in electron acceptors within the photosynthetic pathway (Kautsky et al., 1960). When photosystem II (PSII) absorbs light and electrons (originated from the splitting of water, H2O) are accepted into the electron transport chain, it cannot accept other electrons until the first ones are passed further on to other carriers in the chain. During this period, the reaction center of PSII is considered 'closed.'
A fraction of closed reaction centers results in a temporary reduction in photochemical efficiency, which is followed by a corresponding increase in fluorescence yield (remember the three fates). In dark adapted plants, non-photochemical quenching (NPQ) is initially absent, as these processes take time to activate. Hence, fluorescence yield reaches a maximum. Once NPQ mechanisms start to engage, fluorescence yield decreases and eventually stabilizes at a steady state, the timing of which varies among plant species.
This reaction makes it possible to quantify the maximum potential photosynthetic efficiency of plants, as well as the actual efficiency of photosynthesis under light, and heat dissipation. The interesting thing is that fluorescence yield also changes when plants experience stress. Some PSII centers may become inactive or impaired, altering the fluorescence dynamics.
In future blogs, we will delve into how fluorescence is measured using various methods and explore the key parameters that can be derived from these signals.

